India's ISO 13485 Certification
ISO 13485 certification
Two things cannot be compromised in medical devices:
1. Product quality
2. Safety when using such devices
Therefore, the regulatory standards for such devices are particularly severe and must be followed throughout the medical device's life cycle. Many organizations must demonstrate that they can manage medical equipment quality. This is shown through ISO 13485:2016 certifications.
Medical device quality management systems are governed by ISO 13485.
If you want this certification, contact Certpedia to apply.
Benefits of ISO 13485:2016
1. Medical equipment quality is optimal.
2. This certification will gain medical industry trust.
3. Company credibility and image will improve.
4. The number of satisfied customers will increase.
5. Quality management for medical devices is enhanced.
6. Employees will want to improve.
Criteria for ISO 13485 Certification in India
You must follow the ISO 13485 standards before you can apply for ISO 13485 certification. In order to do that, you should train your organization based on this standard.
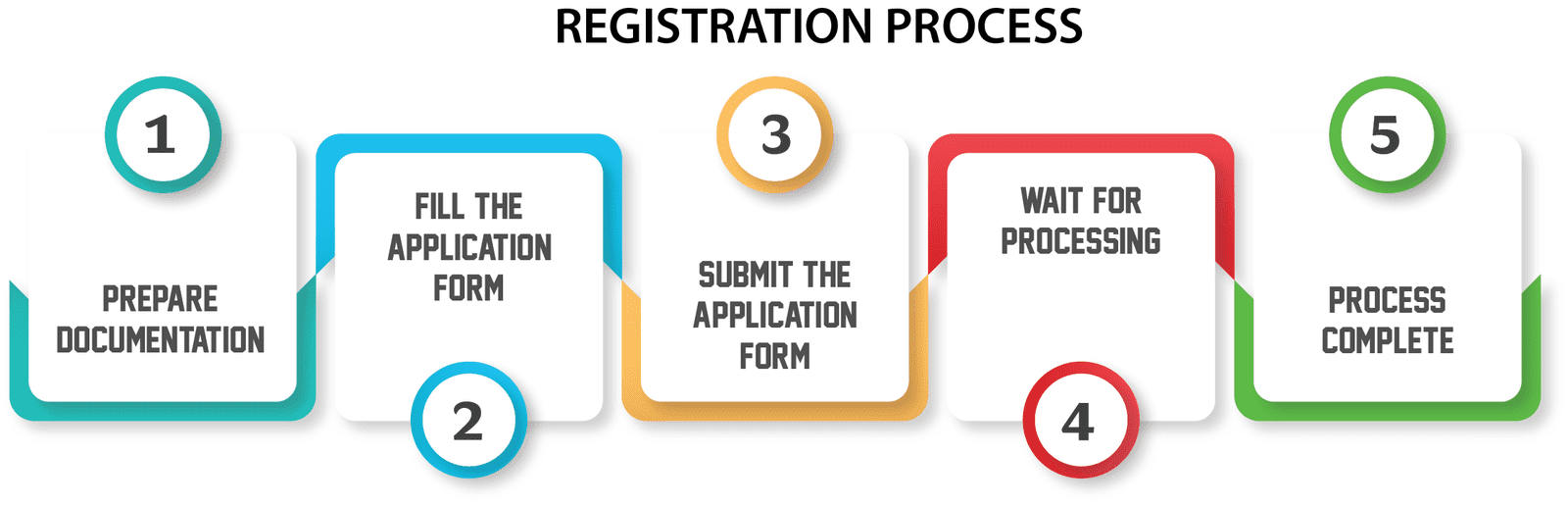
In India, How to Obtain ISO 13485 Certification
1. Getting the papers together
2. Sending in the application for ISO 13485 certification
3. Letting an auditor look at the application and the process document
4. Get the certificate if the auditor says you should.
Certpedia will help you file and register your business and give you the certification.
We'll help you get ISO 13485 certification in India.
Certpedia can help you sign up by giving you the following services:
1. Choose the type of certification.
2. Document aggregation
3. Draft approval
4. ISO 13485 Certification
Your business can use less energy if you make changes. With the assistance of Certpedia, you can obtain ISO 13485 certification to demonstrate that your medical devices are in good working order.